Optimisation of Vaccine Manufacturing and Supply Chain

The FVMR Hub has been researching innovative vaccine technologies and manufacturing processes to inform the manufacturing of future vaccine designs since 2018. The Hub has been outstanding in impacting the field of vaccine manufacturing, particularly in 2020. The Hub is funded by a £10 million grant from the Department of Health and Social Care to pursue vaccine research that impacts Official Development Assistance demographics (often known as lower- and middle-income countries, LMICs). The Hub consists of several UK universities, including the University of Bristol, the University of Nottingham, the University of Cambridge, and King’s College London; UK-based research institutes such as the National Institute for Biological Standards and Controls (NIBSC), the Centre for Process Innovation (CPI), and the NHS Blood and Transplant. The Hub also collaborates directly with international vaccine manufacturers or research institutes in ODA countries, such as Incepta (Bangladesh), Vabiotech (Vietnam), Aimei Hissen (Dalian, China), Hilleman Laboratories (India), and theUganda Virus Research Institute (UVRI, Uganda), as well as the Developing Country Vaccine Manufacturing Network (DCVMN), which comprises over 40 vaccine manufacturing companies from developing countries.
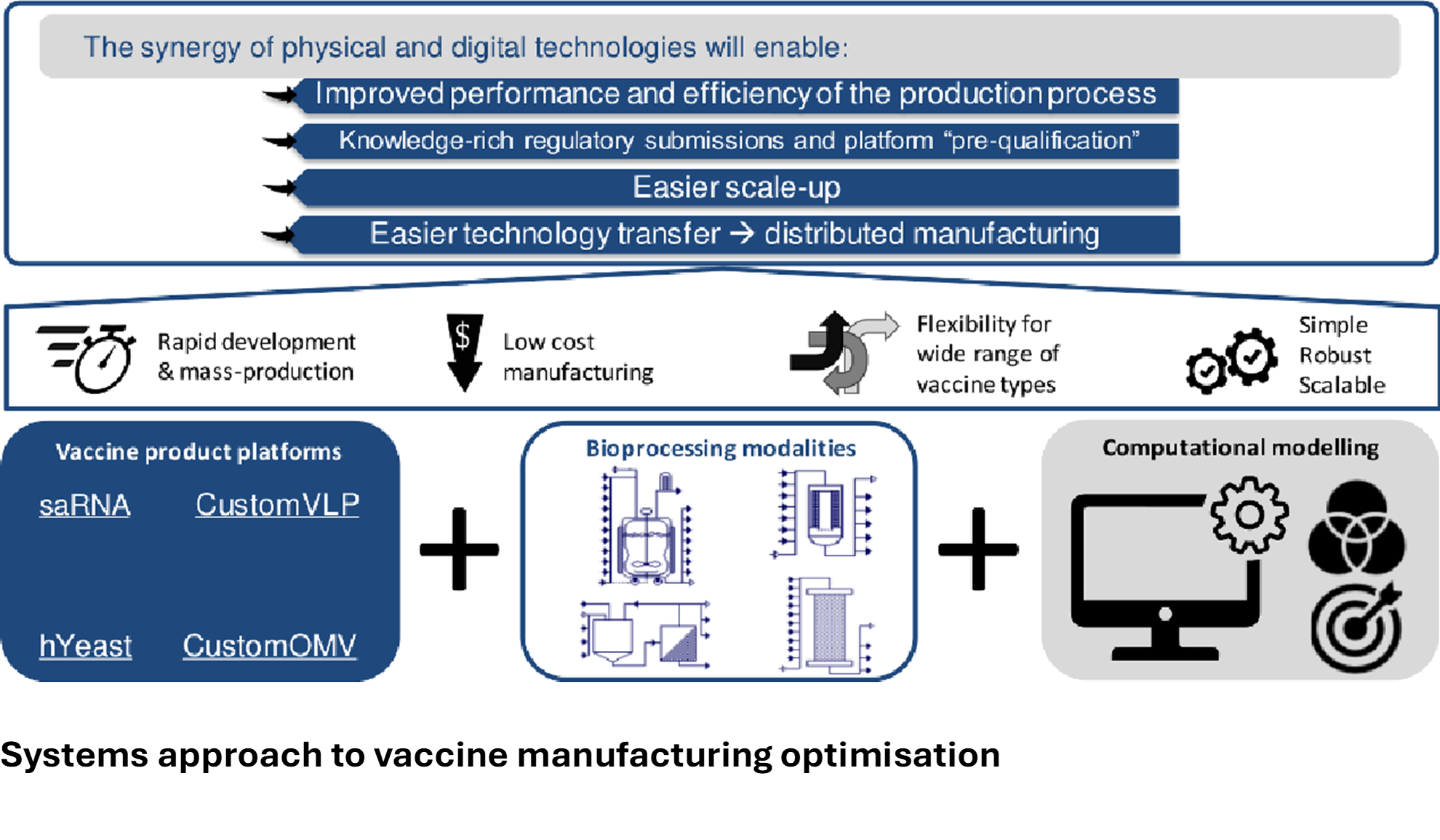
The Hub team researches front-to-back vaccine supply chain issues, supported by modelling and cost analyses; quality control issues arising from innovative vaccine technologies; and innovative formulation strategies focusing on 4 pillar platform technologies: RNA vaccines, yeast-derived vaccines, baculovirus, and bacteria-derived outer membrane vesicles (GMMA).
More than 20 pathogens are targeted by the Hub, including SARS-CoV-2 (causative agent of COVID-19), human papillomavirus, rabies, polio, cholera (cause of most epidemics in the decade prior to 2017), chikungunya virus, zika virus, and pneumococcal disease, amongst others. Each of these pathogens severely affects the economic and health welfare of many of the globe’s population, particularly residents of developing countries.
The outbreak and global pandemic of COVID-19 in early 2020 exemplifies the need for more rapid vaccine manufacturing and proves the impact of innovative vaccine technologies, such as RNA [1]. The FVMR Hub has directly been called on since the outbreak of COVID- 19 to continue and expand upon business-as-usual activities, by focusing resources to support the Imperial self-amplifying RNA vaccine, engaging with the UK’s Vaccine Task Force and MHRA (UK regulatory body) to assess and evaluate the saRNA COVID-19 vaccine candidate, supporting a clinical trial on a COVID-19 RNA vaccine candidate planned in Uganda, informing the world media on supply-chain issues and potential resolutions, informing the world media on COVID-19 vaccines and other vaccines, and training early career researchers as well as vaccine manufacturing staff from developing countries.
The team has modelled and optimised vaccine supply chains in developing countries, such as Kenya [2]. In in a follow-up work, the team quantified the resources, production scales and time required for producing RNA vaccines for the global pandemic demand, as well as the investment in manufacturing required to meet global demand for COVID-19 vaccines [1]. Such thought leadership on emerging technologies for low‐cost and rapid vaccine manufacture and supply was acknowledged through the team’s involvement in the Royal Society’s highly influential DELVE initiative and >50 media interviews globally.
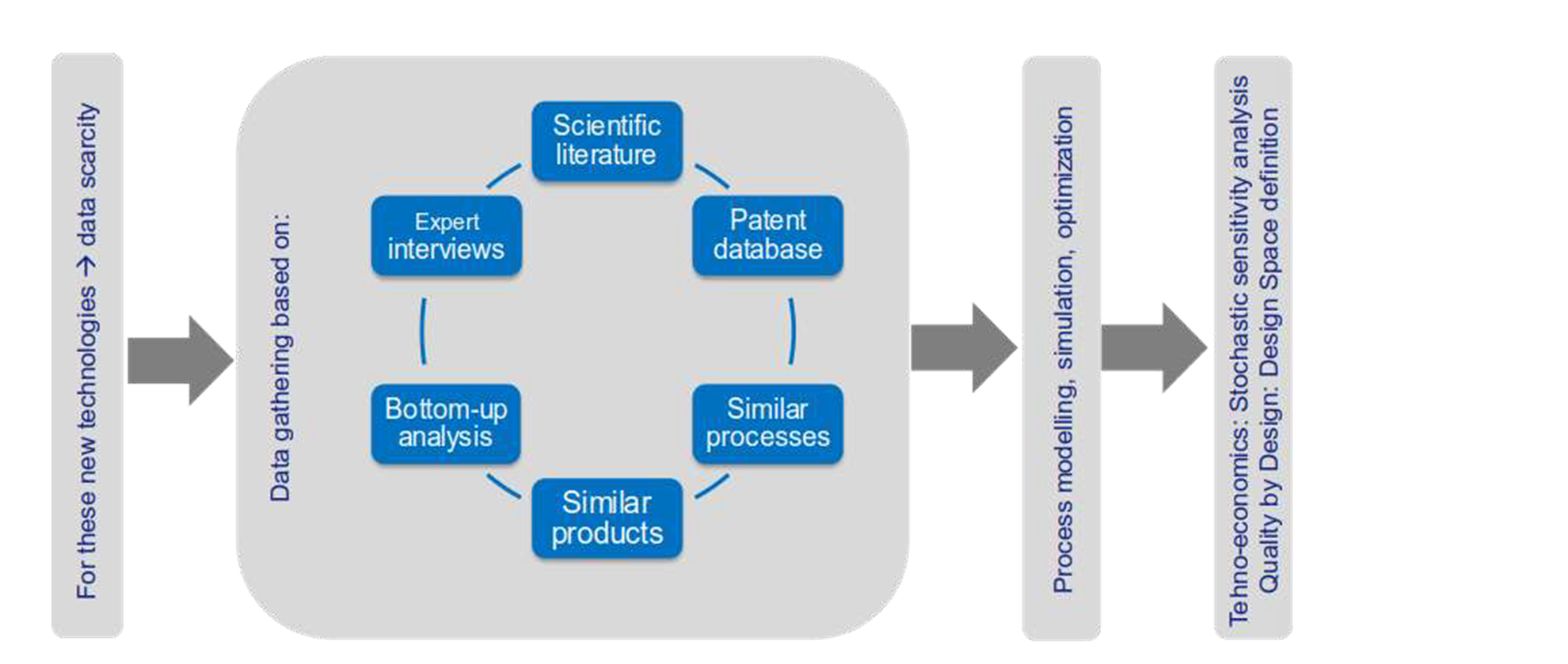
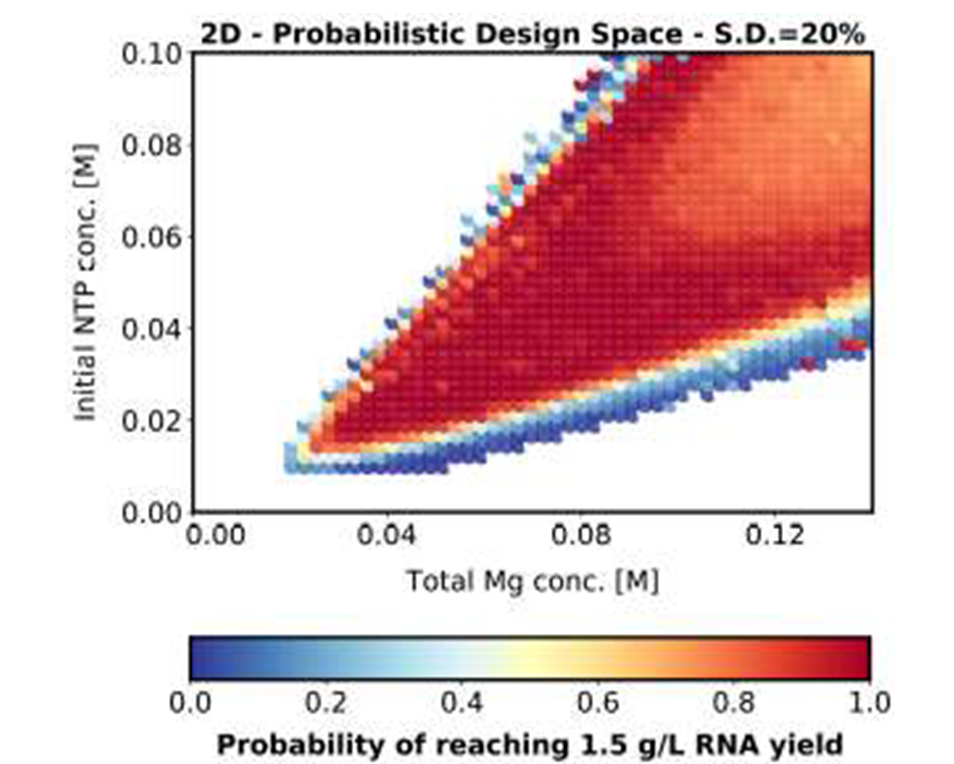
In addition to supply chain issues for RNA vaccines, quality control requires research to optimize this for broader vaccine production. Currently, quality control assessment is conducted using assays that can be refined to provide more detailed information on RNA integrity and quality. Modelling and optimisation including analysis of uncertainty can be used to identify effcient areas of operation which are highly likely to achieve product meeting target quality specifications – the “design space” [3,4]. Importantly, the template of the saRNA vaccine, supported by the newfound knowledge on dosing and reactogenicity, may be progressed to target other pathogens, including those present in developing regions as well as Disease X – the next pandemic.
Our themes

AI & Digital Tech

Energy

Healthcare

Manufacturing

Resilience

Sustainability
View our annual reports
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Curabitur condimentum tempor bibendum. Mauris vel velit viverra, efficitur erat vitae, ullamcorper dolor. Aliquam mattis dignissim sodales. Phasellus accumsan tristique cursus. Donec nunc enim, semper a aliquam ut, sodales in dolor.
